Calculating Exact Masses
The exact mass of a molecule is also called the monoisotopic mass. In brief, it is calculated by adding the exact masses of the most abundant isotopes of the constituent elements. (Please note the difference between isotopic mass and atomic weight!)
For the most common elements found in organic molecules (C, H, N, O) this is a rather straightforward exercise with easily applied results. Take, for example, the case of theobromine (molecular weight 180.17):
| Structure | Atom | # | Mass | Sum |
|---|---|---|---|---|
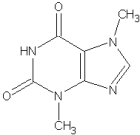 | C | 7 | 12.0000 | 84.0000 |
| H | 8 | 1.0078 | 8.0624 | |
| N | 4 | 14.0031 | 56.0124 | |
| O | 2 | 15.9949 | 31.9898 | |
| Formula: C7H8N4O2 | Exact mass | 180.0646 | ||
For small molecules, the difference between molecular weight and exact mass is slight in the absence of elements having more than one significant isotope. For elements having more than one significant isotope, or larger numbers of an element having a not-so-significant isotope (e.g., carbon) the difference will be greater.
Your structure-drawing software may be able to calculate exact mass for you. There are various software tools online which can also be used.
Here is a link to the SIS Isotope Distribution Calculator and Mass Spec Plotter. Note that the calcualtor assumes neutral charge.